Gas Service
Explosive Limits and Influencing Factors
"Urban Gas Engineering Basic Terminology Standard" GB/T 50680-2012
2.2.8 Explosive Limits
The range of combustible gas volume fraction that can explode when a mixture of combustible gas and air encounters an ignition source.
2.2.9 Upper Explosive Limit
The highest volume fraction of combustible gas when a mixture of combustible gas and air explodes.
2.2.10 Lower Explosive Limit
The lowest volume fraction of combustible gas when a mixture of combustible gas and air explodes.
Urban gas, when leaked into the environment and mixed with air, has the potential to combust or explode when the gas concentration is within the range of the lower explosive limit (LEL) and upper explosive limit (UEL). The explosive limits of gas are not fixed values; they vary due to factors such as gas properties and external conditions. Understanding the influence of internal and external conditions on explosive limits provides valuable reference values for measurements or calculations under specific conditions. The main influencing factors are as follows:
(1) Type and Chemical Properties of Gas
The molecular structure and reactivity of combustible gases affect their explosive limits. The explosive limits are also related to the thermal conductivity (thermal diffusivity) coefficient; higher thermal conductivity leads to a wider explosive range.

Note: From this table, it can be seen that the explosive limits range of 5% to 15% represents the lower and upper explosive limits of methane under conventional conditions. It's important to note that different sources may provide slightly different data. Natural gas typically consists mainly of methane, but it can also contain other components with different explosive limits. Therefore, the correct description would be that the explosive limits of natural gas, depending on its composition, are approximately in the range of 5% to 15%.
(2) Purity of Combustible Gas
The purity of combustible gas affects its explosive limits. An increase in the inert gas content in combustible gas will narrow the range of explosive limits. When the inert gas content increases to a certain value, the mixed gas will no longer undergo explosions.
(3) Uniformity of Gas-Air Mixture
When combustible gas is thoroughly and uniformly mixed with air, if the gas concentration at a certain point reaches the explosive limit, the gas concentration throughout the entire mixed space will also reach the explosive limit. Combustion or explosive reactions occur simultaneously throughout the mixed gas space without interruption, resulting in a larger explosive limit range. When mixing is uneven, certain points within the gas mixture may have gas concentrations at or above the explosive limit, while other points may have concentrations below the explosive limit. This leads to an interruption in combustion or explosive reactions, resulting in a smaller explosive limit range.
(4) Form, Energy, and Location of Ignition Sources
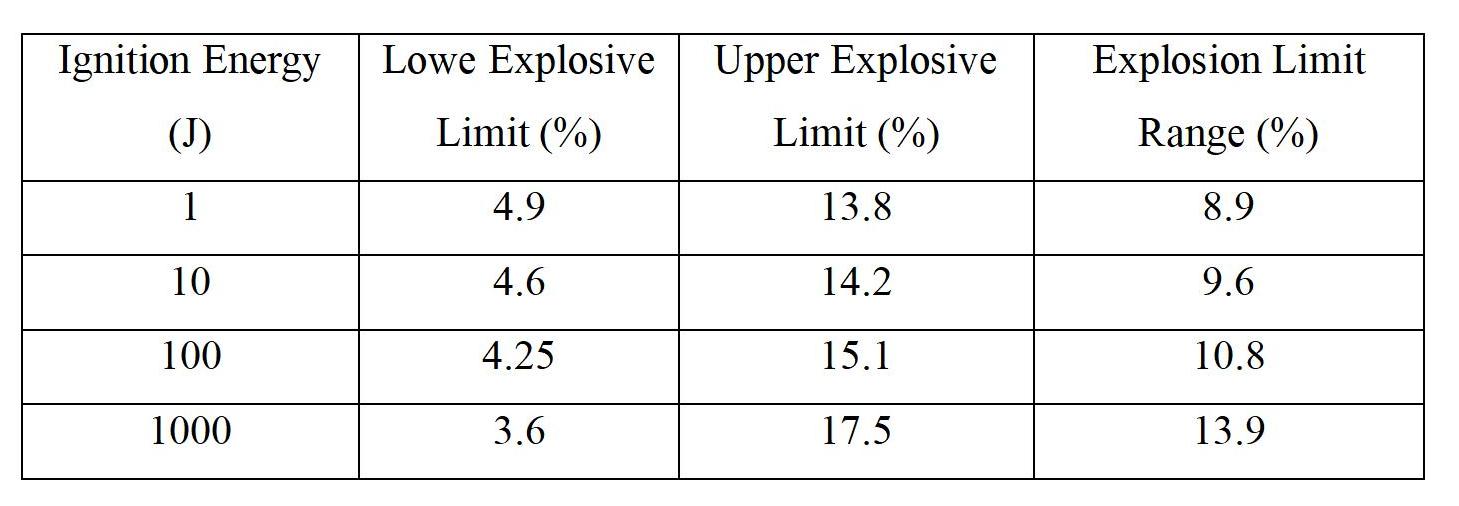
The nature of ignition sources influences the range of explosive limits. Greater energy intensity, larger heating area, longer duration, and ignition sources located closer to the center of the gas mixture will result in a wider explosive limit range. Different ignition sources have varying ignition temperatures and energies. For instance, open flames have higher energy levels compared to typical spark sources, leading to a larger corresponding explosive limit range. Conversely, high-energy spark sources may have a smaller range if they are not continuous. The table below illustrates the impact of ignition energy on the explosive limits of methane (natural gas). As ignition energy increases, the explosive range significantly expands.
(5) Geometric Shape and Size of Explosion Container
The explosive limits of combustible gases are measured through containers, and the size and geometric shape of different test containers, as well as the thermal conductivity of the wall materials, affect the magnitude of the measured explosive limits of the gas. The influence of container size on explosive limits can be explained by the wall effect. Combustion results from a series of chain reactions initiated by free radicals. Combustion or explosive reactions can only occur when the quantity of free radicals produced exceeds the quantity that disappears. If the container has a large surface area and the wall material has a high thermal conductivity coefficient, resulting in significant external heat loss, a greater amount of energy is required to sustain combustion or explosive reactions. Additionally, the probability of free radicals colliding with the container wall decreases, which promotes free radical production, leading to a smaller explosive limit range. Conversely, if the container has a small surface area, and the wall material has a low thermal conductivity coefficient, resulting in minimal external heat loss, less energy is needed to sustain combustion or explosive reactions. The likelihood of free radicals colliding with the container wall increases, hindering free radical production, resulting in a larger explosive limit range. Currently, there are various methods for testing the explosive limits of combustible gases, including spherical sealed containers, cylindrical containers, and open-ended glass tube tests. Different testing methods and conditions may yield slightly different explosive limits for the same combustible gas. The testing method for determining the explosive limits of combustible gases in air should follow the current national standard "Methods for Determination of the Explosive Limits of Combustible Gases in Air" GB/T 12474, which specifies the measurement of explosive limits for combustible gases.
(6) Temperature and Pressure of Combustible Gas-Air Mixture
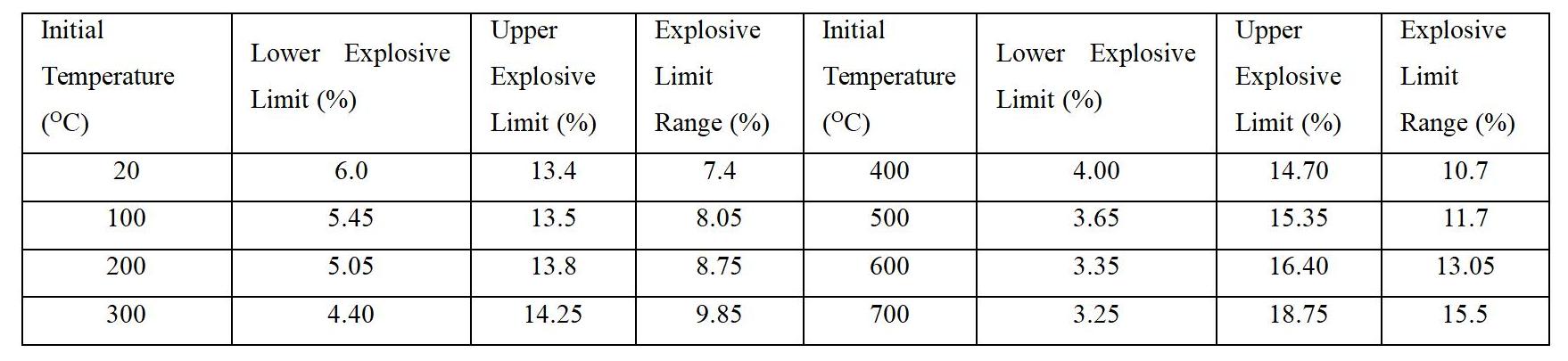
1) Temperature. Raising the temperature of combustible mixtures accelerates combustion or explosive reactions, increasing the reaction temperature and expanding the explosive limit range. Therefore, higher temperatures increase the explosiveness of combustible gas mixtures. The table below provides a set of data illustrating the impact of initial temperature on the explosive limits of natural gas mixtures.
2) Pressure. Increasing the pressure of flammable mixtures reduces the inter-molecular spacing, increases collision probability, accelerates reaction rates, widens the explosive range, and significantly alters the upper explosive limit. The explosive limit range expands. The table below illustrates the effect of initial pressure on the explosive limits of methane. In general, as the initial pressure rises, the upper explosive limit noticeably increases. However, among known flammable gases, only carbon monoxide exhibits a reduction in explosive limits with increasing initial pressure.
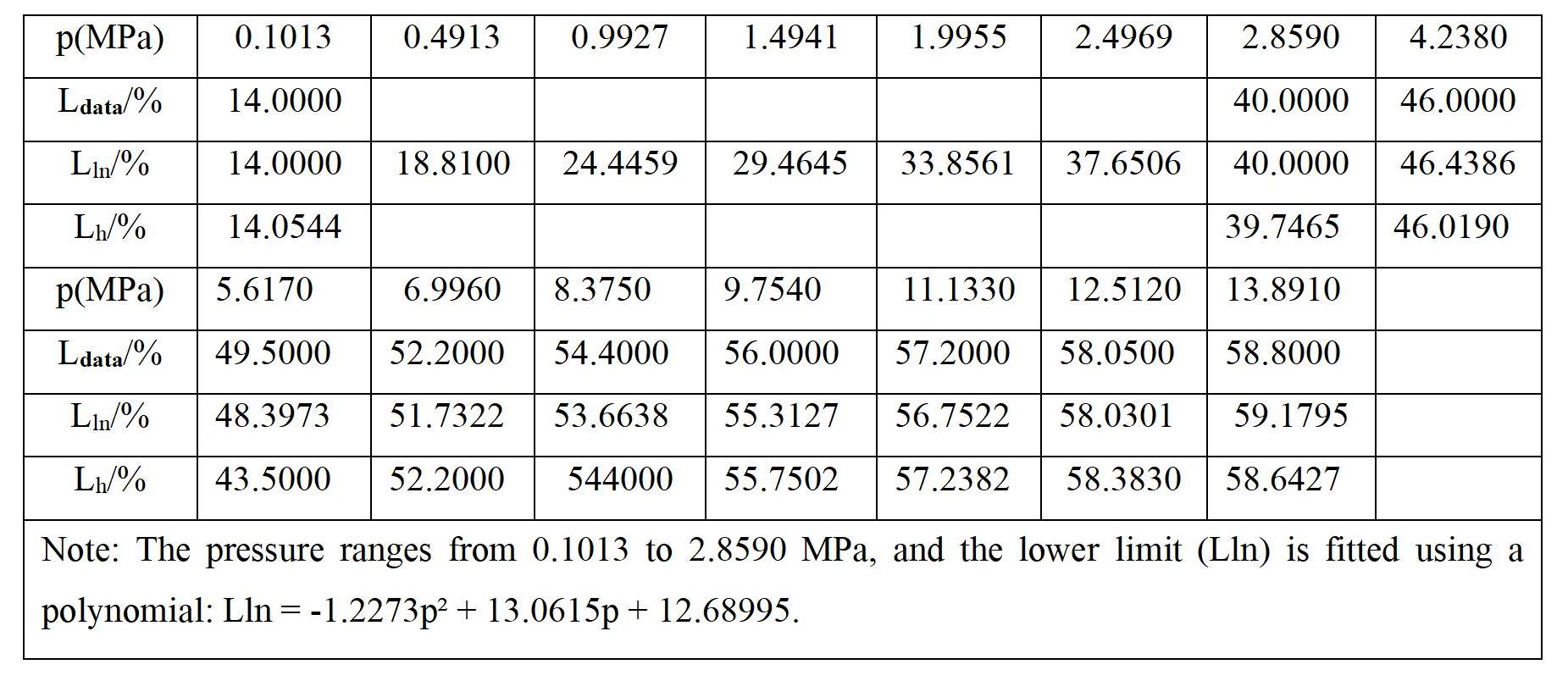
A decrease in initial pressure results in a reduction of the explosive limit range. When the initial pressure decreases to a specific value, the upper and lower explosive limits coincide. At this point, the pressure is referred to as the explosive critical pressure. Systems operating below the explosive critical pressure do not explode. Therefore, reducing pressure within sealed containers is advantageous for safety.
The size of the explosive limit range, including the upper and lower limit data, holds significant guidance for gas operation management. Subsequently, we will compile the numerical values for their practical applications in daily work.